Scientific Library: results
Publications & Application notes
Validation of a Monocyte Activation Test (MAT) using the PyroMAT™ kit
Download the posterDevelopment of orthogonal chromatographic methods for the purity analysis of therapeutic oligonucleotides
Download the posterAbsolute Quantification of Oligonucleotides by ICP-MS/MS
Download the posterConference

ICP MS/MS: An innovative approach for the characterisation of therapeutic oligonucleotides
ICP-MS/MS: An Innovative Approach for the Characterisation of Therapeutic Oligonucleotides
ICP-MS/MS methodologies were developed for the characterisation of therapeutic oligonucleotides:
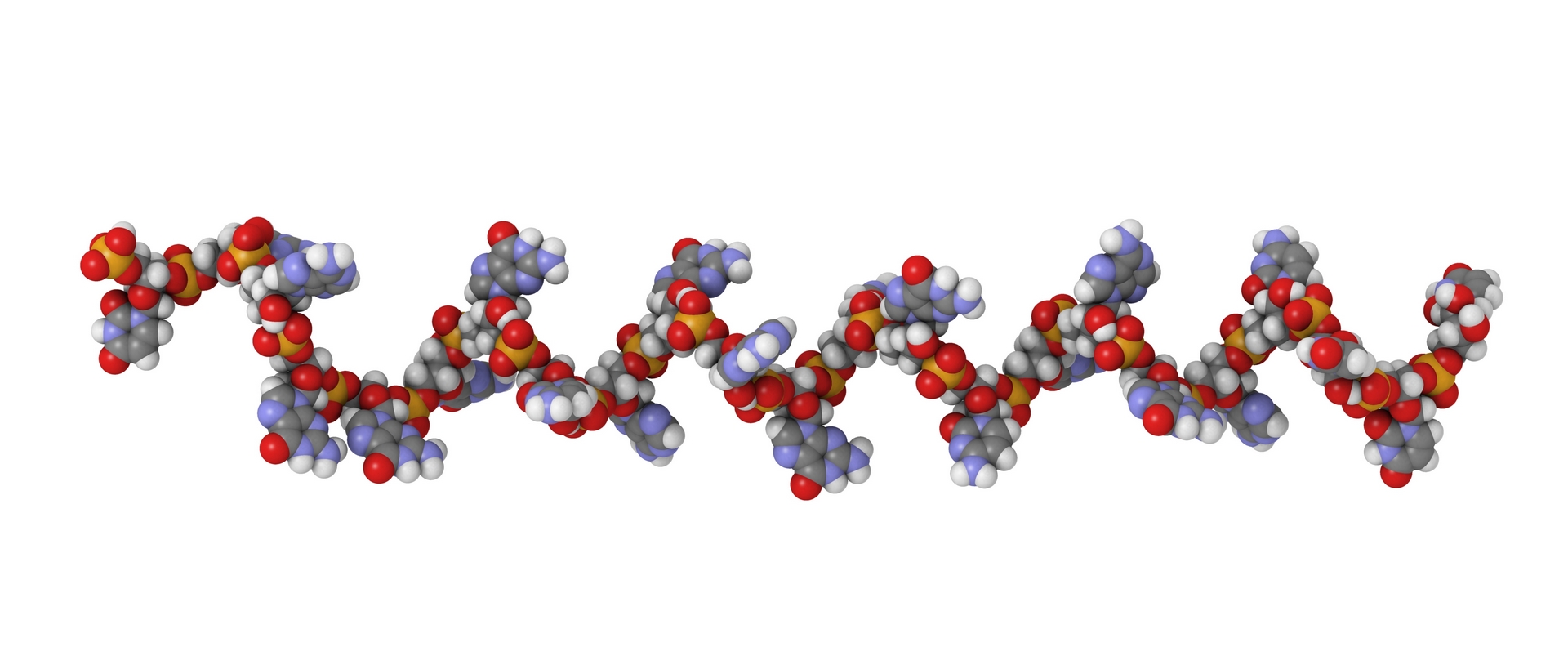
Characterisation of therapeutic oligonucleotides by ICP-MS/MS
Quality Assistance developed an innovative method for the absolute quantification of therapeutic oligonucleotides, as well as for the determination of phosphodiester-to-phosphorothioate (PO/PS) ratio by ICP-MS.MS.
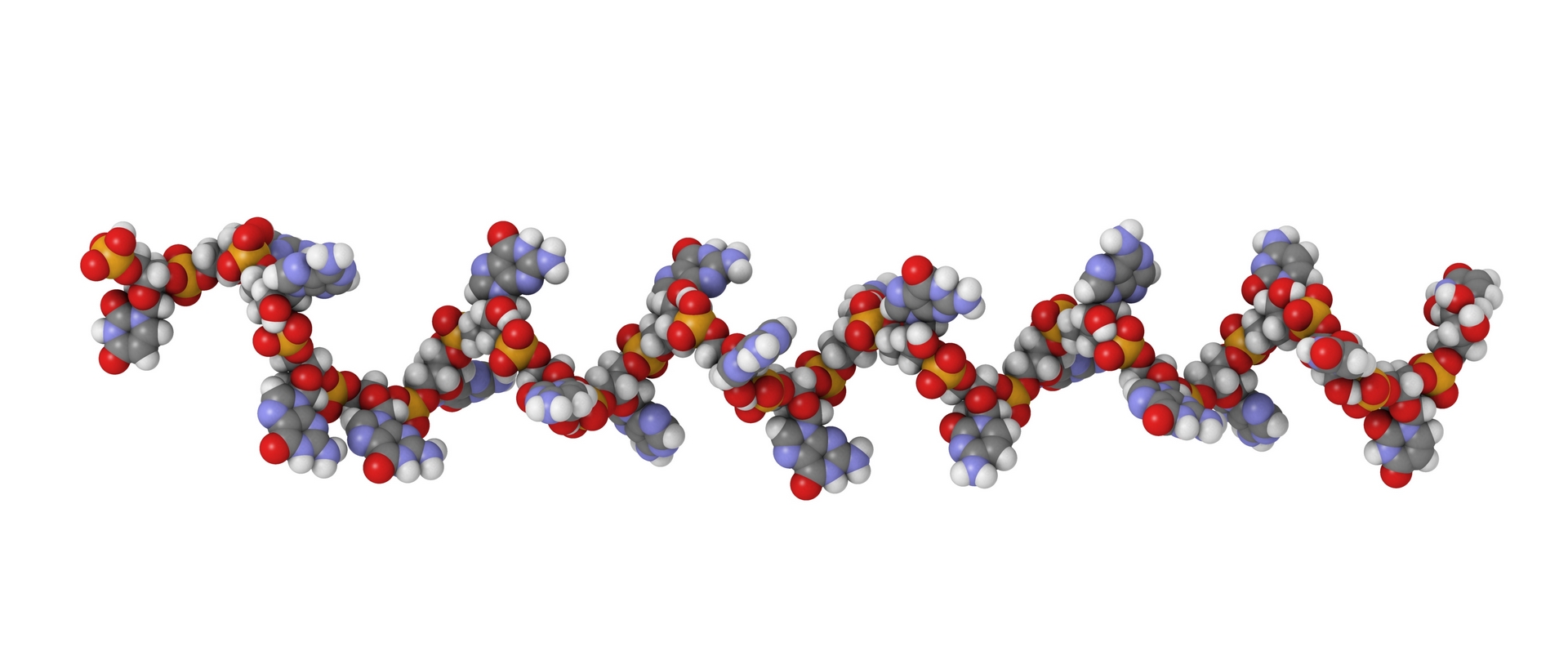
Oligonucleotides : absolute quantification and P=O/P=S ratio in a single ICP-MS/MS run
ICP-MS can be a solution for a fast and accurate generic method for oligonucleotides quantification without the reference oligo !
Download the slides
Webinar
Excipient & API suppliers: ICH Q3D is also for you!
Access this 30 minute webinar to learn how the Q3D guideline for EIs can be efficiently implemented for all drug substances, excipients and drug products.
Download the slides
Elemental Impurities: How to be compliant with Q3D ?
Access this webinar which presented how the Q3D guideline for EIs can be efficiently implemented for all drug substances, excipients and drug products.
Download the slides
Scientific poster
Elemental impurities: how to be compliant with ICH Q3D guideline?
Location
CPhI ISCE 2015


