Scientific Library: results
Publications & Application notes
Robustness evaluation of weak anion exchange chromatography method for the purity analysis of therapeutic oligonucleotides
Download the posterAnalysis of Oligonucleotides by Size-Exclusion Chromatography: SEC-UV, SEC-MS, SEC-MALS
Download the posterValidation of a Monocyte Activation Test (MAT) using the PyroMAT™ kit
Download the posterDevelopment of orthogonal chromatographic methods for the purity analysis of therapeutic oligonucleotides
Download the posterAbsolute Quantification of Oligonucleotides by ICP-MS/MS
Download the posterInnovative ICP-MS/MS approach for the determination of phosphodiester to phosphorothioate ratio in phosphorothioate oligonucleotides
Download the posterWebinar

Analysis of Oligonucleotides by Size-Exclusion Chromatography: SEC-UV, SEC-MS, SEC-MALS
The field of therapeutic oligonucleotides has seen remarkable progress over the last years.
OLIGONUCLEOTIDES: absolute quantification and P=O/P=S ratio in a single ICP-MS/MS run
Access this 20 minutes webcast to learn how Quality Assistance developed an ICP-MS/MS method based on the quantification of P and S after microwave digestion to determine the P=O/P=S ratio in oligonucleotide samples.
Download the slides
Excipient & API suppliers: ICH Q3D is also for you!
Access this 30 minute webinar to learn how the Q3D guideline for EIs can be efficiently implemented for all drug substances, excipients and drug products.
Download the slides
Elemental Impurities: How to be compliant with Q3D ?
Access this webinar which presented how the Q3D guideline for EIs can be efficiently implemented for all drug substances, excipients and drug products.
Download the slides
Conference

ICP MS/MS: An innovative approach for the characterisation of therapeutic oligonucleotides
ICP-MS/MS: An Innovative Approach for the Characterisation of Therapeutic Oligonucleotides
ICP-MS/MS methodologies were developed for the characterisation of therapeutic oligonucleotides:
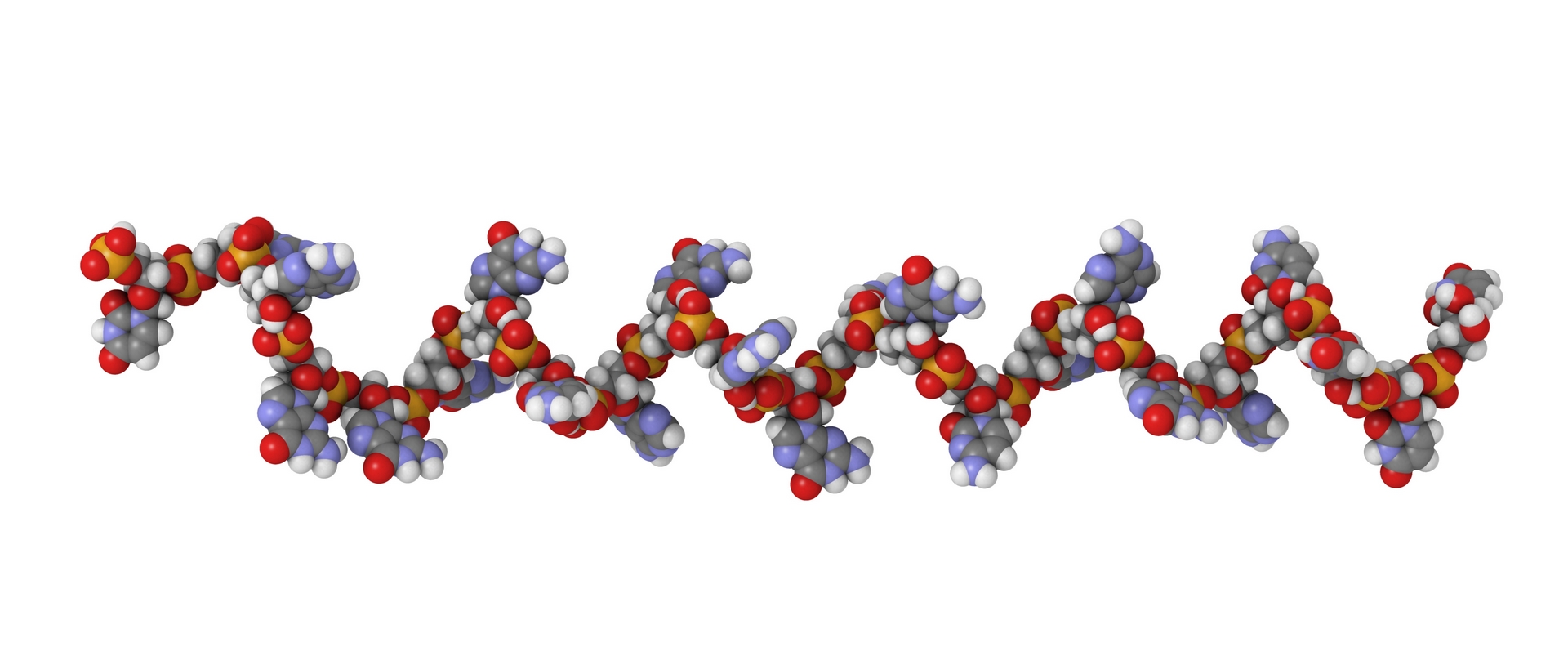
Characterisation of therapeutic oligonucleotides by ICP-MS/MS
Quality Assistance developed an innovative method for the absolute quantification of therapeutic oligonucleotides, as well as for the determination of phosphodiester-to-phosphorothioate (PO/PS) ratio by ICP-MS.MS.
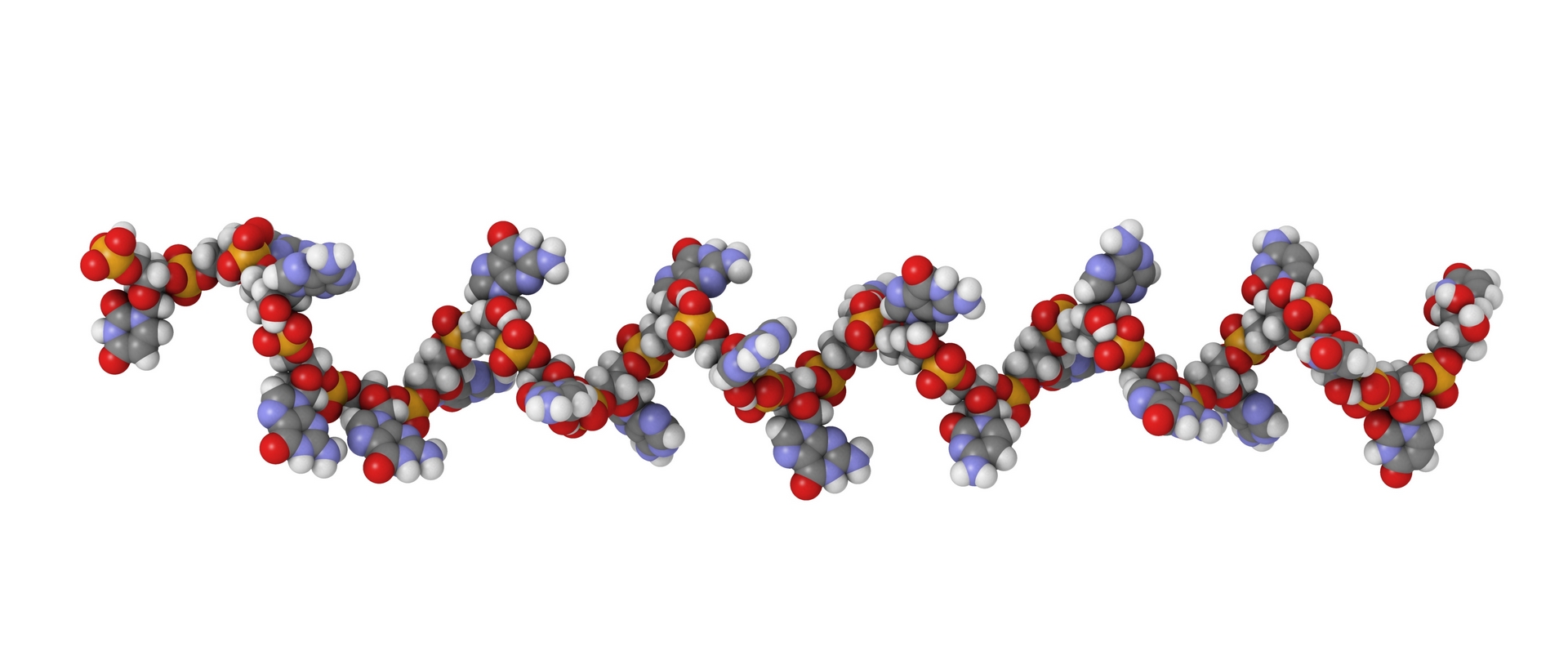
Oligonucleotides : absolute quantification and P=O/P=S ratio in a single ICP-MS/MS run
ICP-MS can be a solution for a fast and accurate generic method for oligonucleotides quantification without the reference oligo !
Download the slides
Scientific poster
Development of orthogonal chromatographic methods for the purity analysis of therapeutic oligonucleotides
Download the posterOligonucleotides: Absolute quantification and P=O/P=S in a single ICP-MS/MS run
Location
TIDES 2018
Elemental impurities: how to be compliant with ICH Q3D guideline?
Location
CPhI ISCE 2015



