Automated electrophoresis platform TapeStation 4150
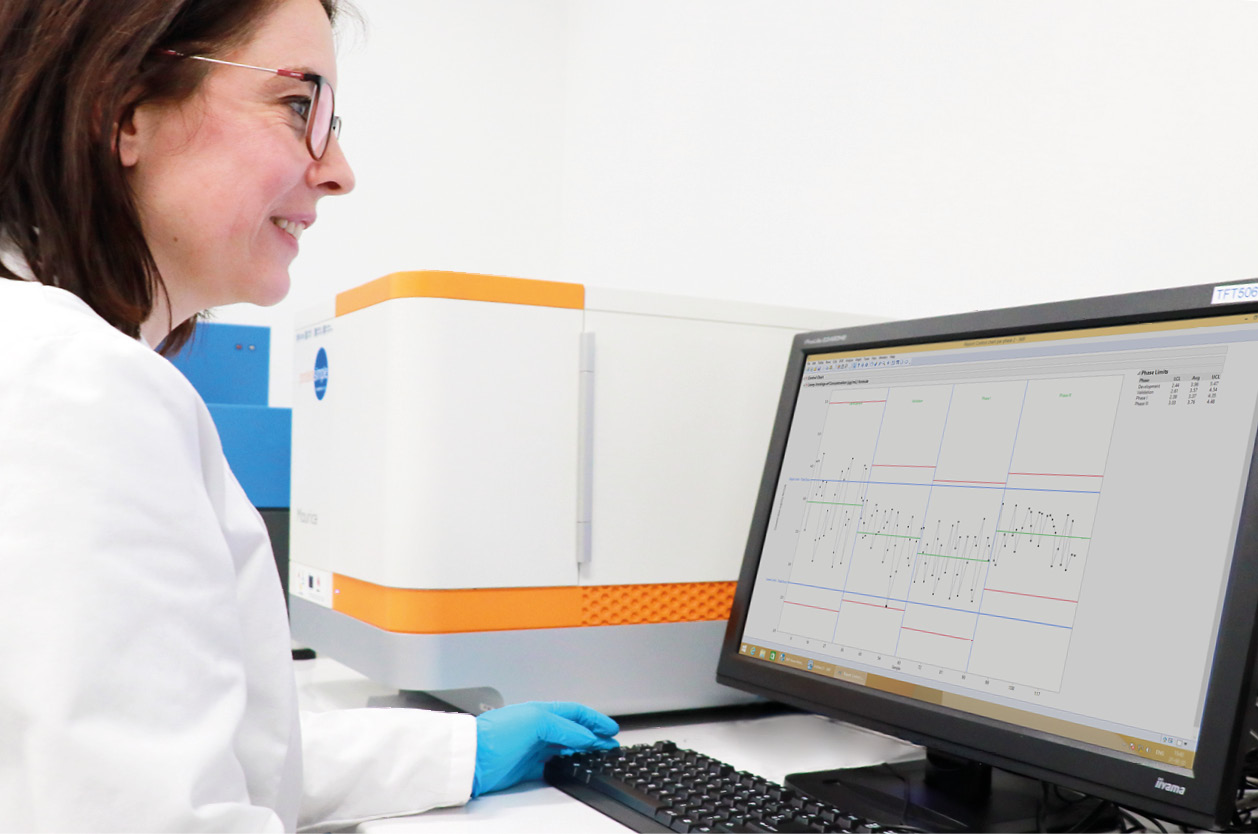
The TapeStation 4150 system (Agilent) is an automated electrophoresis platform designed for the quality control of DNA and RNA

The TapeStation 4150 system (Agilent) is an automated electrophoresis platform designed for the quality control of DNA and RNA

The MiniSeq System is a benchtop-sized Next Generation Sequencing (NGS) platform from Illumina that
Andrew+ (Waters) is a fully automated pipetting robot for sample preparation.

The MauriceFlex system, from Bio-Techne, is a capillary electrophoresis
The AID (Autoimmun Diagnostika) MultiSpot reader is a multifunctional imaging device designed for the analysis of enzymatic and fluorescent (FluoroSpot) based ELISpot assay
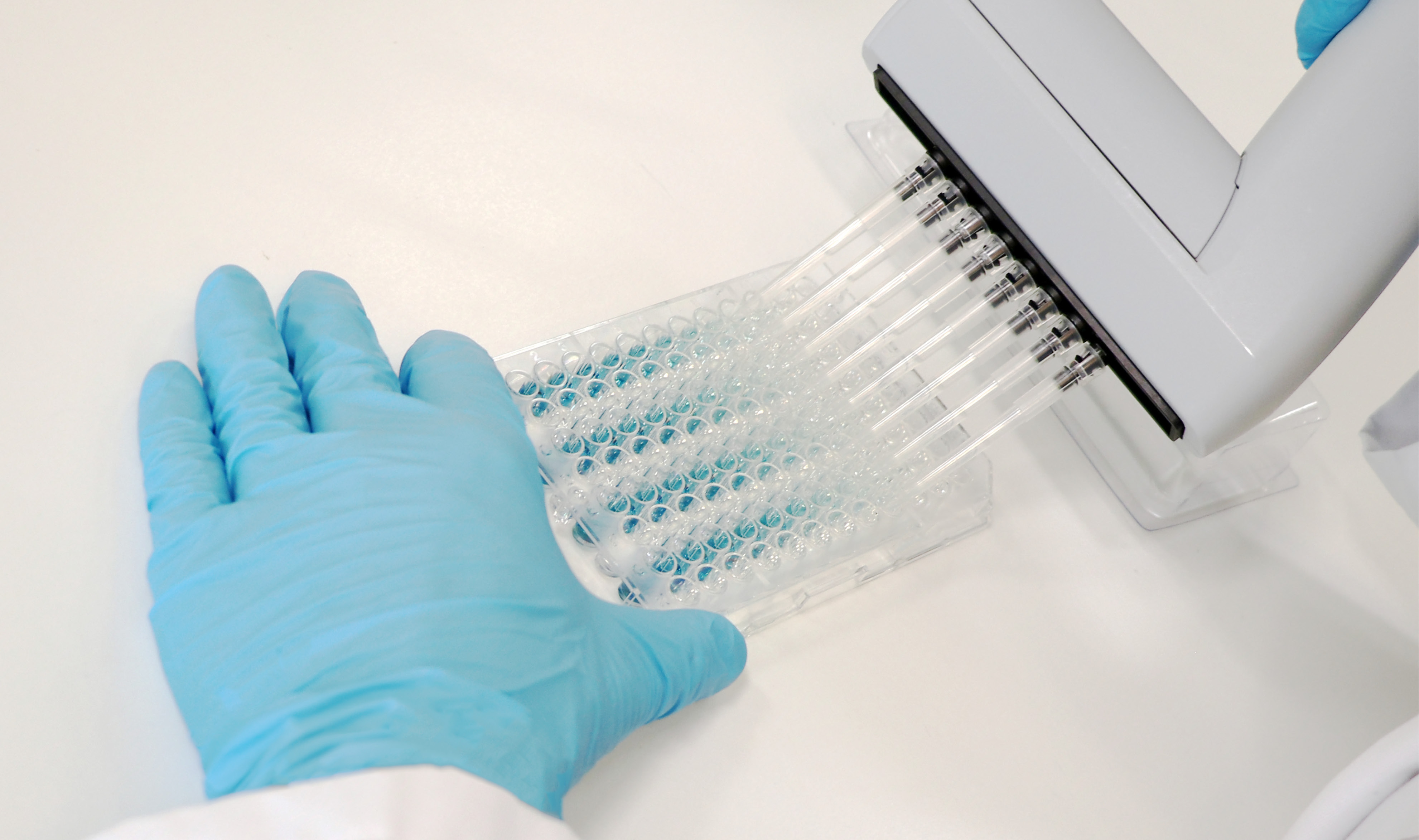
Bacterial endotoxins and pyrogens (both endotoxins and non-endotoxin pyrogens (NEPs) from bacteria, viruses, yeasts, and molds) are critical quality attributes (CQ

The autoflex maX is a matrix-assisted laser desorption ionisation (MALDI) with tandem Time-of-Flight (TOF/TOF) mass spectrometer from

Organosilicon-based compounds such as PDMS (low-molecular weight polydimethylsiloxane, i.e., Dimeticone, Simeticone, Antifoam A®
Multi-Angle Light Scattering (MALS) detection allows for the determination of molecular weight distribution of biomolecules after size-exclusion chromatography (S
The KingFisher Flex is a device that automatically processes nucleic acids, proteins or cell samples.

PCR (Polymerase Chain Reaction) is a method used to quantify nucleic acids.

The 2D UPLC/MS system allows the online combination of two chromatographic columns with hyphenation to mass spectrometry.


The SoloVPE technology (Variable Pathlength Extension) offers a reliabl

Our latest FACS model features 6 active lasers and can perform up to 21 color marker analysis, allowing us to reduce cross talk and spectral overlap