You’re going to love our pleasant workplace located in the countryside, surrounded by woods, fields and birds.

You’re going to love our pleasant workplace located in the countryside, surrounded by woods, fields and birds.

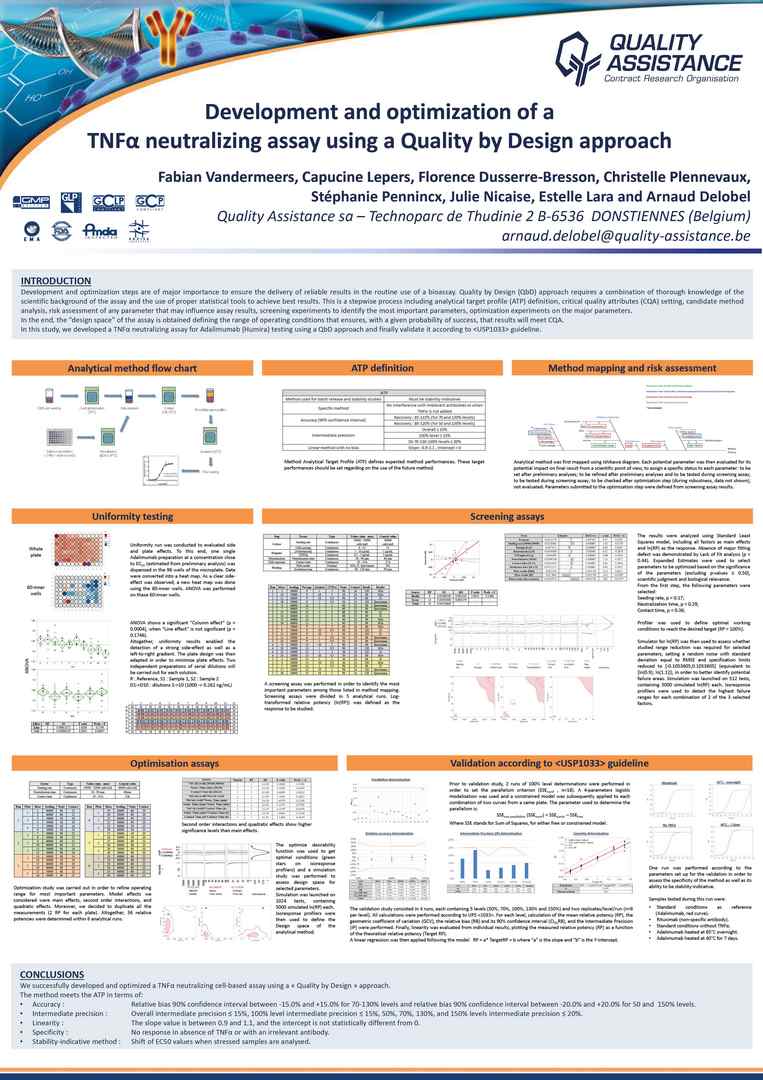
Development and optimization steps are of major importance to ensure the delivery of reliable results in the routine use of a bioassay. Quality by Design (QbD) approach requires a combination of thorough knowledge of the scientific background of the assay and the use of proper statistical tools to achieve best results.

Ensuring the characterisation of in vitro differentiated cells for therapeutic purposes brings many challenges. These include the difficulty of controlling cell variability and complexity of the steps required to generate a stable product. All the analytical assays required to characterise a Cell-Based Medicinal Product (CBMP) also have to meet several requirements described in different regulatory guidances.1,2 The aim of this study was to develop and validate analytical methods required for identity, purity and biological activity assessment for CBMPs.
Cell therapy medicinal products are complex products. Most of the time, the correct mechanism of action and the composition of the product are not completely known. Robust, precise and accurate validated methods are then needed to characterise this kind of products in terms of identity, purity and potency.
The discovery of human pluripotent stem cells 10 years ago turned the spotlight on the potential of pluripotent stem cells for personalised cell therapy. The scientific interest then quickly shifted towards the use of these cells for safety pharmacology, drug discovery and disease modelling. For all these purposes, in the mid to long term, properly characterised cell banks will be necessary.
The characterisation of embryonic (ESC) and induced pluripotent stem cells (IPSC) used for manufacturing requires the development and validation of analytical methods (e.g. flow cytometry, microscopy, QPCR and bioassays). Cell characterisation includes the testing of cell product identity, determination of impurities, and assessment of biological activity and viability. Among the techniques available, flow cytometry is widely used to assess the expression of cell markers. Our laboratory has developed flow cytometry panels dedicated to the characterisation of extracellular and intracellular markers of ESC and IPSC, and to the detection of cell-related impurities. We proposed a method for the validation of flow cytometry panels according to the recommendations of international guidelines on the validation of analytical methods.
IPSC differentiated into cardiomyocytes and MSC-like cells were also used to test the performance of our flow cytometry panels to accurately monitor the manufacturing process of cell products.
In addition to the technical tips, this webinar aims at presenting a critical view on the use of flow cytometry platform for cell characterisation.
KEY LEARNING OBJECTIVES
Access this 45 minutes webinar to learn how to characterise pluripotent stem cells in terms of identity and purity. The analytical performance of the methods developed have been discussed along with the presentation of experimental results obtained during method development, optimisation and/or validation.
WHO WILL BENEFIT?
in the biopharmaceutical industry, involved in:
EXPERTISE & CAPACITY
We provide a complete dedicated service:
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) is a mechanism of action of antibodies through which virusinfected or cancer cells are targeted for destruction by components of the cell-mediated immune system, such as natural killer cells. For most monoclonal antibodies targeting cell-surface antigens, ADCC is usually the mechanism that better reflects the in vivo mode of action of the drug. For this reason, quality control assays based on ADCC activity are required. In this application note, the use of a commercial kit commercialised by Promega to determine ADCC activity of anti-CD20 antibodies is presented. Complementary methods based on binding properties by Surface Plasmon Resonance and on the determination of the N-glycosylation profile by high-resolution mass spectrometry are also discussed.
ADCC activity is one of the main mechanisms of actions of therapeutic monoclonal antibodies used for oncology indications. The activity of these antibodies can be studied in vitro using a reporter-gene cell-based assay. This test is usually part of the control strategy of the drug substance and drug product.
In some cases, surrogate tests can be used. Binding to FcγRIIIa receptor using Surface Plasmon Resonance is one of these tests. Additionally, the study of antibody core fucosylation can provide useful information, e.g. to interpret differences that may arise in terms of in vitro ADCC activity.

Although drug products issued from biotechnology have now been marketed for many years, accurate protein quanti fi cati on remains a challenge.

A large number of pharmaceuti cals are glycosylated proteins. An adequate glycosylati on is criti cal for therapeuti c proteins in terms of safety, bioacti vity, solubility, stability, and pharmacokinetics and dynamics.